CYP1A2 (Cytochrome P450 1A2)
thingiverse
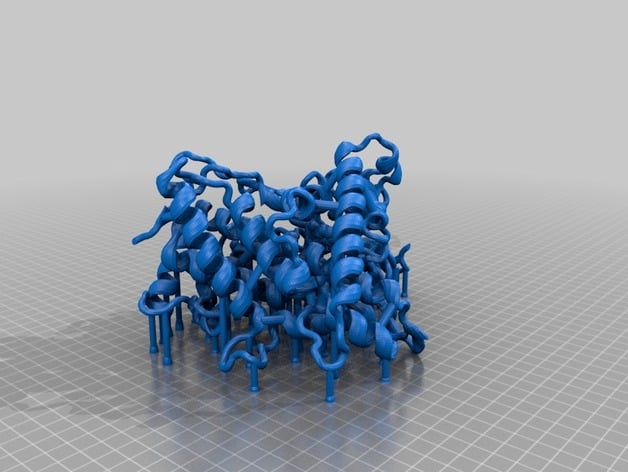
Imaged is my first printout of a PDB 2HI4 molecule cross-section (Cytochrome P450 1A2, or CYP1A2 for short, complex with alpha-napthoflavone ligand). A cross-section rendition was chosen to facilitate identification of the active site, heme cofactor, ligand, and highlighted amino acids. What is CYP1A2 and why is it important? CYP1A2 is a member of the cytochrome P450 enzyme family. It is involved in the metabolism of many different drugs, such as the blood thinner Clopidogrel (trade name: Plavix) and everyone's favorite, caffeine! Non-synonymous mutations (also known as missense mutations) occur as a result of small nucleotide polymorphisms (SNPs). Missense SNPs within the gene encoding for CYP1A2 can modulate the protein's. Missense SNPs in CYP1A2 are found in different frequencies across populations and can be used as bio-markers for physicians. DNA sequencing technology can allow physicians to know a patient's genetic makeup at many different genes. By knowing a patient's bio-markers for gene encoding for the CYP1A2 protein, physicians can determine drug prescription dosages that are just right for that individual based on what their genetic bio-markers say. This is the basis of pharmacogenomics. What's special about this model?: I have highlighted in this model a few amino acids of interest. These amino acids are rendered in CPK format: 1). rs28399418 marks a missense SNP at the 314th amino acid (Ile314Val). This amino acid residue is located within the active site. Furthermore, the isoleucine residue is highly conserved between many mammals. This suggests that Ile314 plays an important role in CYP1A2's activity. This residue is included in the model and is located near the heme cofactor. It is the amino acid not bound to the heme cofactor. 2). rs28399424 marks a missense SNP at the 431st amino acid (Arg431Trp). This amino acid residue is located outside of the active site. The BLOSUM substitution score for this substitution is -2 and the arginine residue is also highly conserved between mammals. This suggests that Arg431 most likely plays a key role in maintaining proper structure of the protein. A tryptophan substitution most likely will disrupt the proteins structure at this site given that arginine (positively-charged and hydrophillic) and tryptophan (bulky and hydrophobic) are very biochemically different. In the model, Arg431 is the only amino acid highlighted that lies outside of the active site in the periphery. 3). The final highlighted amino acid is the 314th amino acid which is a cysteine residue. Cys314 closely interacts with the heme cofactor. It is likely that mutation from this cysteine to any other amino acid would greatly alter the activity of CYP1A2. In the model, this is the only amino acid that touches the heme cofactor. It should be noted that mutations of the highlighted residues have not been studied experimentally for clinical relevance so no firm conclusions can be made. The conclusions made above are conjectures based off of fundamental knowledge of amino acids, protein structure, and conservation evolution. This model was made as a teaching tool for providing visual insight on missense SNP mutations and their applications into the evolving field of pharmacogenomics. References: 1) Henikoff, S., & Henikoff, J. G. (1992). Amino acid substitution matrices from protein blocks. Proceedings of the National Academy of Sciences of the United States of America, 89(22), 10915–10919. http://doi.org/10.1073/pnas.89.22.10915 2) UCSC Genome Browser: Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Res. 2002 Jun;12(6):996-1006. http://genome.ucsc.edu/ 3) PDB ID: 2HI4 Sansen, S., Yano, J. K., Reynald, R. L., Schoch, G. A., Griffin, K. J., Stout, C. D., & Johnson, E. F. (2007). Adaptations for the oxidation of polycyclic aromatic hydrocarbons exhibited by the structure of human P450 1A2. Journal of Biological Chemistry, 282(19), 14348–14355. http://doi.org/10.1074/jbc.M611692200 Print Settings Printer Brand: MakerBot Printer: MakerBot Replicator (5th Generation) Rafts: Yes Supports: Yes Notes: Must be printed with both rafts and supports (may take up to 40+ hours using the print settings specified). Post-Printing Reccomendations The printout is relatively fragile in the non-secondary structure regions (the regions that are not alpha helices or beta sheets). I ended up breaking a few small sections of the model on accident while removing the support scaffolding after printing. I have a few suggestions: 1). Be patient: the amount of support scaffold that is printed out is quite extensive and will take a few hours to remove it all properly. 2). Be delicate: the model is semi-robust. Some regions are quite can be dealt a good amount of punishment before breaking while other regions can break off using finger force. The two suggestions are especially true for removing the scaffolding within the active site and the deep interior of the molecule! Removal of the scaffolding in this area is made much easier with precision tools, a little bit of dexterity, and patience. The use of long-skinny needle nose pliers is highly recommended! How I Designed This The original PDB file (2HI4: http://www.rcsb.org/pdb/explore.do?structureId=2hi4) was manipulated using VMD (http://www.ks.uiuc.edu/Research/vmd/) and BIOVIA Discovery Studio (http://accelrys.com/products/collaborative-science/biovia-discovery-studio/). BIOVIA Discovery Studio was used to produce a cross section of CYP1A2 and identify various residues inside and outside of the active site. VMD was used to render the CYP1A2 cross-section in "New Cartoon" format and the highlighted amino acids, heme cofactor, and alpha-napthoflavone molecules in "CPK" format. VMD was then used to export the modified molecule as an .STL file. The exported .STL file was imported into Tinkercad (https://www.tinkercad.com/). Within Tinkercad, reinforcing braces and supports were added manually. The angled supports found within the molecule are meant to be left in to provide structural integrity to the model, whereas the vertical supports found underneath the model are mean to facilitate the printing process.
With this file you will be able to print CYP1A2 (Cytochrome P450 1A2) with your 3D printer. Click on the button and save the file on your computer to work, edit or customize your design. You can also find more 3D designs for printers on CYP1A2 (Cytochrome P450 1A2).
